Variables associated with remission in spinal surgical site infections
Introduction
Surgical site infections (SSIs) are a feared complication of spine surgery. They are associated with enhanced morbidity (1), increased costs and prolonged hospital stay for the patients (2). Although relatively frequent, few studies address remission associated to surgical debridement, patients’ co-morbidities and modalities of antibiotic use. Most papers principally investigate the epidemiology of SSIs of the spine (3,4), with an overall incidence oscillating between less than 2% (5) to 15% (3), rather than its treatment success. Another subset of papers reveals innovative surgical techniques in the therapy of these infections. Literature focusing on antibiotic regimens in spinal SSI is even sparser (3,5). Most publications or experts recommend a minimum length of intravenous antibiotic courses of 4–6 weeks, often followed by prolonged oral antimicrobial regimens (3,6-9), or they do not pronounce on detailed antibiotic modalities at all (10-12). Most of these recommendations are reviews and lack own data (6-9). Any benefits of long-term antibiotic use remain to be determined (3). Other recent options and recommendations include hyperbaric oxygen, negative-pressure vacuum wound therapy (13-15), closed suction irrigation systems (2,16), or local antibiotic use (17). However, comparative data supporting these individual recommendations are lacking. This is a single-centre study over eight years; we analyze variables associated with long-term remission with an emphasis on surgical and antibiotic-related parameters. Of note, we do not assess variables with the occurrence of SSIs after spine surgery, which the reader may consult specific literature (11,12,18).
Methods
We performed a single-centre case-control study, involving adult patients who were hospitalised in our Bone Infection Unit (BIU) and underwent a surgical spinal procedure and antibiotic therapy for a SSIs of the spine between January 2007 and December 2014. We included only deep infections accompanied by a combination of pus, growth of bacteria from intraoperative specimens, and a minimal follow-up of 6 months. For clarity, we excluded cases with community-acquired infections, superficial scar infections, paediatric cases, spine infections after surgeries performed elsewhere in the spine, or insufficient information regarding surgical and antibiotic treatment, fungal or mycobacterial infections, and those SSIs treated by surgeons alone, e.g. without concomitant Infectious Diseases consultations.
For each episode of infection, we assessed variables pertaining to demographic characteristics, immune suppression status, microbiology results, surgical procedures, antibiotic treatment, and various outcomes. We defined remission as the absence of clinical, laboratory or imaging evidence of recurrence of the original infection. A surgeon (JB) and an Infectious Diseases physician (IU) independently recorded each variable on an Excel® spreadsheet and resolved any disagreements by consensus. All microbiological were based on the Clinical and Laboratory Standards Institute guidelines (19). To enhance specificity, we only accepted cultures with growth on agar plates, and did not consider growth in enrichment broth. Patients were followed-up to 30 June 2015.
Statistical analyses
We performed group comparisons using the Pearson-χ2-test, the Fisher-exact, or the Wilcoxon-ranksum-test, as appropriate. Cox regression analyses with cluster-control (random-effect at patients’ level) determined associations with the outcome remission. We introduced independent variables with a P value ≤0.05 in univariate analysis stepwise into the multivariate analysis, except for variables for surgical interventions and antibiotic treatment, which we automatically took into the final model. We included 5 to 8 predictor variables per outcome (20) and checked key variables for collinearity and interaction (by Mantel-Haenszel estimates and interaction terms). We equally plotted recurrence against the antibiotic duration to detect visually eventual thresholds above which remission could be enhanced. We used STATA software (9.0, STATA™, USA). P values ≤0.05 (two-tailed) were significant.
Results
Study population
We revealed 66 episodes of postoperative spine infections in 48 different patients, of which 49 episodes (74%) were related to spinal instrumentation. Fourteen patients revealed two episodes, and others three four episodes. These patients had a median age of 65 years (range, 18–88 years); 27 episodes (41%) occurred in women, and 22 (33%) in immune-compromised patients (diabetes mellitus in 12 cases, immune-suppressive therapy in 8 cases, cirrhosis CHILD C in 1 case, and metastatic cancer in 1 case). The median body mass index was 25.4 kg/m2. Six patients yielded peripheral arterial diseases, and only 10 were active current smokers. The surgical indications for initial surgery were various: 9 episodes of fracture, 2 cases of cancer, and 15 episodes of disc herniation. Overall, in 39 episodes, the spine was described as degenerative, with spinal cord compression in 26 cases, paraplegia in 6 episodes, and sagittal malalignment in 11 cases. Initial surgery involved bone graft in 21 cases, and cement in 7 cases. Two surgeries were followed by therapeutic irradiation. The surgical access had been performed by anterior approach in 57 (86%) episodes.
Infection
Infection was polymicrobial in 19 episodes (29%), and associated to increased pain in 34 cases, purulent discharge in 41 episodes, and metalwork loosening in 7 cases. Among 30 different microbiological patterns, the four most predominant pathogens were methicillin-susceptible Staphylococcus aureus (n=21), S. epidermidis (n=8), methicillin-resistant S. aureus (n=5), and Klebsiella pneumoniae (n=5). There was no particular outbreak situation among our patients during the study period. The median serum C-reactive protein level at admission was 85 mg/L (range, 1 to 500 mg/L). The median serum leukocyte count was 8.8 G/L (range, 4.6–17.5 G/L).
Therapy
All episodes were operated for infection with a median of 2 surgical interventions (range, 1 to 10 interventions; 11 episodes with complete metalwork removal), and negative pressure wound therapy in 28 (27%) cases. Irrigation systems were not used. The median duration of antibiotic therapy was 8 weeks (range, 1 to 52 weeks), including 2 weeks of intravenous treatment (range, 0 to 18 weeks) and 8 episodes with local antimicrobial therapy (bacitracin in 7 cases, and combined bacitracin and vancomycin in 1 episode). Concerning systemic antibiotic therapy, we recorded 37 different intravenous regimens and 19 various oral prescription patterns. Cefuroxim, vancomycin, and amoxicillin-clavulanate were the most used agents, combined with rifampicin in 34 infections. The median follow-up period after therapy was 2.6 years (range, 0.5 to 6.8 years).
Outcomes
Overall, in 13 (20%) of the episodes were clinical recurrences with a median of 2 months after completion of previous treatment. However, 9 of these 13 recurrences (69%) yielded the same pathogen, whereas we have found a new pathogen in the four other cases suggesting either selection by previous therapy or a new infection. Conversely, 53 episodes were in remission (80%). The patients who had clinical failure and those who did not were similar regarding key variables (Table 1).
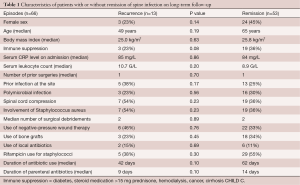
Full table
Multivariate adjustment
In cluster-controlled multivariate Cox regression analysis adjusting for the case-mix, no variable was significantly associated with “remission”. Especially, the following factors were not significantly related to remission: number of surgical interventions [hazard ratio (HR) 0.9; 95% confidence interval (CI), 0.8–1.1]; infection due to Staphylococcus aureus (HR 0.9; CI, 0.8–1.1), local antibiotic therapy (HR 1.2; CI, 0.6–2.4), and, duration of total (HR 1.0; CI, 0.99–1.01) (or just parenteral) (HR 1.0; CI, 0.99–1.01) antibiotic use (Table 2). Receiving antibiotic therapy for <6 versus >12 weeks had the same risk of clinical recurrence (χ2 test; P=0.90). Similarly, there was no minimal threshold, beneath which the recurrence risk increased significantly. A recurrence occurred equally despite total antibiotic prescription over 80 days or parenteral administration of 33 days. Our results were similar to our literature selection (Table 3).
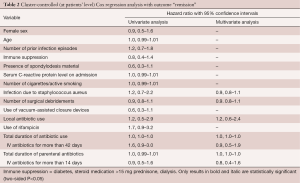
Full table
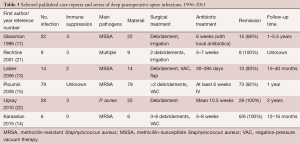
Full table
Discussion
Our main interest was factors related to persistent remission after treatment of a SSIs of the spine. Our overall remission rates were consistent with the literature yielding a wide range of remission incidence ranging from 68% to 99% after the first therapeutic approach (3-5) (Table 3). In contrast, we failed to reveal any parameters of antibiotic therapy or surgical treatment predicting clinical failure or remission. Specifically, after a minimal follow-up of 6 months there was no evidence that remission was related to the number of surgical debridement, the use of a negative-pressure therapy device, administration of a parenteral antibiotic regimen or the total duration of antibiotic therapy.
As in many field of septic orthopaedic surgery, the number of surgical debridement does not formally influence remission rates, which has been shown for chronic osteomyelitis (23), septic native joint arthritis (24), fracture-device infections (25), infected open fractures (26), or prosthetic joint infections (27), while all available literature bases on retrospective studies. To the best of our knowledge, there is no randomized prospective trial targeting the number of debridement for outcome remission. There is very little evidence to guide surgical treatment of patients who require a single versus multiple debridement (28). Dipaola et al. developed a predictive model by a multivariate logistic regression model for spinal SSIs basing on 128 infected patients from their centre. Among 30 clinical variables analyzed, and despite the retrospective nature of their analysis, they have validated four variables being strongly predictive regarding the necessity of multiple debridement: infection due to methicillin-resistant S. aureus, bacteraemic disease, posterior lumbar spine and use of non-autograft bone grafts (28). Due to the small sample size for each of these particular variables, we could not confirm or reject their model. Equally, the use of a negative pressure vacuum therapy did not yield benefice in terms of long-term remission. The literature is emerging when it comes to negative-pressure therapy, especially in orthopaedic or plastic surgery. Different authors reported a positive effect of negative-pressure vacuum therapy of spinal SSI, but again none of the studies are comparative (13-15).
The most important message of our study lays in the antibiotic part. We think that none knows the ideal duration of intravenous or total antibiotic therapy, which moreover might be patient or pathogen-dependent. Most author groups report a minimum length of parenteral antibiotic courses of 4–6 weeks and a total duration up to three months (3,6-9,15,17,21), although some authors only recommend 2 weeks of parental therapy (9,29) or even only 2–3 days (16), without further compromising the success when compared to the literature. To cite examples, Clark and Shufflebarger treated delayed infections with surgery and 48-72 hours of parenteral antibiotics followed by ten days of targeted oral antibiotics. All infections were eradicated (16,30). Likewise, Richards and Emara administered antimicrobials only for three weeks, of which 2–5 days intravenously, followed by a 7 to 14 day-course of oral treatment (16,31). Of note, these cited examples concern mostly paediatric and immune-competent adolescent patients, but confirm the feasibility of shorter regimens for these specific patient populations. To the best of our knowledge, we ignore if other groups equally treated with such short duration among immune-compromised adult and multimorbid patients.
If there were indeed no benefit to long duration antibiotic therapy, it would be important to limit the use of these agents to avoid furthering the problem of antibiotic resistance and adverse events (32). We personally think that, as long as oral antibiotics are used with good bioavailability and bone tissue diffusion (9,32), the antimicrobial treatment can theoretically be oral from the start in absence of bacteraemia or hemodynamically significant sepsis, and should not last more than 6 weeks for infected bone or disc. Glassman et al. successfully treated two patients with spinal SSI with oral ciprofloxacin from the start, an antibiotic with excellent oral bioavailability and bone penetration (17). Similarly, a course for a soft tissue infection probably does probably not need to be longer than two weeks.
Our study has several limitations. It is a retrospective, single-center study in a resource-rich setting (BIU), and involving only 66 episodes, which limits the generalizability of our findings. Likewise, our case-control design was not able to control for some theoretically important parameters, such as the aggressiveness of surgical debridement or bone grafts (2). Moreover, all of our patients underwent surgical debridement and antibiotic therapy. Therefore, we cannot compute the success rates with surgery or antimicrobial therapies alone. We also recognize that we may have missed long-term clinical failures in patients who were treated at another medical centre after their initial surgery at ours. However, because our centre is the largest in the area and the only public hospital, we think it is unlikely that our patients would seek surgical treatment elsewhere. Finally, the retrospective study design raises the risk for “confounding by indication,” i.e., treating physicians might have preferred more debridement, longer antibiotic administration or more vacuum-assisted negative pressure use for difficult cases. This potential bias reinforces the need for randomized, multicentre studies to improve the sample size for stratification analyses.
Acknowledgments
We thank to the teams of Laboratory of Microbiology and Service of Infectious Diseases for their invaluable help.
Footnote
Conflicts of Interest: Parts of the manuscript have been presented as a poster at the Swiss National Meeting of Orthopedic Surgery, in Basel, June 2015.
References
- Faundez AA. Infectious spondylodiscitis: the surgical approach. Rev Med Suisse 2006;2:709-10, 713-4. [PubMed]
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg 1997;86:975-80. [Crossref] [PubMed]
- Awe O, Harrop J. Surgical Site Infection: a Review. JHN Journal 2010;5:28-9.
- Kërveshi A, Halili N, Kastrati B, et al. Local irrigation of the surgical field with antibiotics in the end of procedure reduces the infection rate in herniated lumbar disc surgery. Mater Sociomed 2014;26:398-400. [Crossref] [PubMed]
- Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 2000;13:422-6. [Crossref] [PubMed]
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med 2010;362:1022-9. [Crossref] [PubMed]
- Bible JE, Biswas D, Devin CJ. Postoperative infections of the spine. Am J Orthop (Belle Mead NJ) 2011;40:E264-71. [PubMed]
- Hegde V, Meredith DS, Kepler CK, et al. Management of postoperative spinal infections. World J Orthop 2012;3:182-9. [Crossref] [PubMed]
- Lazennec JY, Fourniols E, Lenoir T, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res 2011;97:S107-16. [Crossref] [PubMed]
- Chaudhary SB, Vives MJ, Basra SK, et al. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med 2007;30:441-51. [PubMed]
- Pull ter Gunne AF, Mohamed AS, Skolasky RL, et al. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976) 2010;35:1323-8. [Crossref] [PubMed]
- Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976) 2013;38:E1425-31. [Crossref] [PubMed]
- Labler L, Keel M, Trentz O, et al. Wound conditioning by vacuum assisted closure (V.A.C.) in postoperative infections after dorsal spine surgery. Eur Spine J 2006;15:1388-96. [Crossref] [PubMed]
- Karaaslan F, Erdem Ş, Mermerkaya MU. Wound management with vacuum-assisted closure in postoperative infections after surgery for spinal stenosis. Int Med Case Rep J 2014;8:7-11. [Crossref] [PubMed]
- Ploumis A, Mehbod AA, Dressel TD, et al. Therapy of spinal wound infections using vacuum-assisted wound closure: risk factors leading to resistance to treatment. J Spinal Disord Tech 2008;21:320-3. [Crossref] [PubMed]
- Gerometta A, Rodriguez Olaverri JC, Bitan F. Infections in spinal instrumentation. Int Orthop 2012;36:457-64. [Crossref] [PubMed]
- Glassman SD, Dimar JR, Puno RM, et al. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine (Phila Pa 1976) 1996;21:2163-9. [Crossref] [PubMed]
- Ishii M, Iwasaki M, Ohwada T, et al. Postoperative deep surgical-site infection after instrumented spinal surgery: a multicenter study. Global Spine J 2013;3:95-102. [Crossref] [PubMed]
- Wikler MA, Cockerill FR, Craig WA, et al. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. Standard M100-S17. 2007.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. [Crossref] [PubMed]
- Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma 2001;15:566-9. [Crossref] [PubMed]
- Uçkay I, Dinh A, Vauthey L, et al. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect 2010;16:353-8. [Crossref] [PubMed]
- Rod-Fleury T, Dunkel N, Assal M, et al. Duration of post-surgical antibiotic therapy for adult chronic osteomyelitis: a single-centre experience. Int Orthop 2011;35:1725-31. [Crossref] [PubMed]
- Uçkay I, Tovmirzaeva L, Garbino J, et al. Short parenteral antibiotic treatment for adult septic arthritis after successful drainage. Int J Infect Dis 2013;17:e199-205. [Crossref] [PubMed]
- Al-Mayahi M, Betz M, Müller DA, et al. Remission rate of implant-related infections following revision surgery after fractures. Int Orthop 2013;37:2253-8. [Crossref] [PubMed]
- Dunkel N, Pittet D, Tovmirzaeva L, et al. Short duration of antibiotic prophylaxis in open fractures does not enhance risk of subsequent infection. Bone Joint J 2013;95-B:831-7. [Crossref] [PubMed]
- Bernard L, Legout L, Zürcher-Pfund L, et al. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect 2010;61:125-32. [Crossref] [PubMed]
- Dipaola CP, Saravanja DD, Boriani L, et al. Postoperative infection treatment score for the spine (PITSS): construction and validation of a predictive model to define need for single versus multiple irrigation and debridement for spinal surgical site infection. Spine J 2012;12:218-30. [Crossref] [PubMed]
- Kuo CH, Wang ST, Yu WK, et al. Postoperative spinal deep wound infection: a six-year review of 3230 selective procedures. J Chin Med Assoc 2004;67:398-402. [PubMed]
- Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1909-12. [Crossref] [PubMed]
- Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine (Phila Pa 1976) 2001;26:1990-6. [Crossref] [PubMed]
- Uçkay I, Jugun K, Gamulin A, et al. Chronic osteomyelitis. Curr Infect Dis Rep 2012;14:566-75. [Crossref] [PubMed]


